The mass of carbon dioxide will be 238.51g
Step-by-step explanation:
Given:
Volume, V = 81.3 L
Pressure, P = 204 kPa
P = 204000Pa
Temperature, T = 95°C
T = 95 + 273K
T = 368K
mass of CO₂, m = ?
According to the gas law:
PV = nRT
where, R is the gas constant
n is the moles
and the value of R = 8.314 X 10³ L⋅Pa⋅K⁻¹⋅mol⁻¹
n = m/w
where,
m is the mass of the substance
w is the molecular weight
and molecular weight of CO₂ is 44 g
On substituting the value we get:
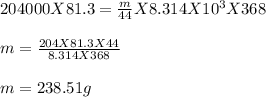
Therefore, the mass of carbon dioxide will be 238.51g