Answer:
1.63 kilo Joules of energy will be produced when 14.8 g of silver nitrate react.
Step-by-step explanation:
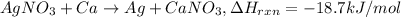
Mass of silver nitrate = 14.8 g
Moles of silver nitrate =
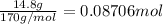
According to reaction, when 1 mole of silver nitrate reacts with 1 mole of calcium it gives 18.7 kJ of heat.
Then amount of heat released when 0.08706 moles of silver nitrate reacts :
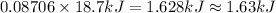
1.63 kilo Joules of energy will be produced when 14.8 g of silver nitrate react.