Answer:
130 gram of NaN₃should be put into the compartment
Step-by-step explanation:
Given that Ideal volume for an airbag = 50 lit
Pressure = 765 mmHg
we know
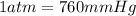
∴
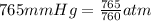
Temperature (T) = 29.5°C = (29.5 + 273) = 302.5 K
We know A/c to ideal gas equation
PV = nRT
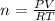
Where n= no. of mole
R = universal gas constant = 0.0821 lit. atm. mol⁻¹K⁻¹
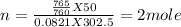
Mass of (NaN₃) = mole x molar mass = 2 x 65 = 130 gram