Answer:
pH = 4.64
Step-by-step explanation:
To find the pH of a buffer solution we need to use the Henderson-Hasselbalch equation:
![pH = pKa + log(([CH_(3)COO^(-)])/([CH_(3)COOH]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/hd5fpicxme46zhywwc0kzmeqej1njjsban.png)
We have:
pKa = 4.76
pH_{i} = 5.00
[CH₃COOH] + [CH₃COO⁻] = 0.100 M
V = 135 mL = 0.135 L
To find the pH after the student adds the solution of HCl, first, we need to find the concentrations of the acetic acid and acetate (conjugate base). To do that we will calculate the number of moles before and after the addition of HCl solution.
Before the addition of the HCl solution we have:
![pH = pKa + log(([CH_(3)COO^(-)])/([CH_(3)COOH]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/hd5fpicxme46zhywwc0kzmeqej1njjsban.png)
![5.00 = 4.76 + log(([CH_(3)COO^(-)])/([CH_(3)COOH]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/m4bcurcrwjm2zjxo2psncyrx58ffntgpsa.png)
![([CH_(3)COO^(-)])/([CH_(3)COOH]) = 1.74](https://img.qammunity.org/2021/formulas/chemistry/high-school/s4a7wnp16av7fzrb7povbz294mtcnbs5s9.png)
![[CH_(3)COO^(-)] = 1.74*[CH_(3)COOH]](https://img.qammunity.org/2021/formulas/chemistry/high-school/7lgo11hceq72xvfnb4ena60qie3ahacgj6.png) (1)
(1)
Also we have that the total molarity of acid and conjugate base in this buffer is 0.100 M:
![[CH_(3)COOH] + [CH_(3)COO^(-)] = 0.100 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/meg7px2v1idv28kjhf097b054w8nuzg16r.png) (2)
(2)
Solving equation (1) and (2) for [CH₃COOH] and [CH₃COO⁻], we have:
[CH₃COOH] = 0.036 M
[CH₃COO⁻] = 0.064 M
Now, the number of moles of the acid and conjugate base is:
![\eta_{[CH_(3)COOH]} = 0.036 mol/L*0.135 L = 4.86 \cdot 10^(-3) moles](https://img.qammunity.org/2021/formulas/chemistry/high-school/oyqxwmdtf6rpx6390ivzc5oaodzocdt48g.png)
![\eta_{[CH_(3)COO^(-)]} = 0.064 mol/L*0.135 L = 8.64 \cdot 10^(-3) moles](https://img.qammunity.org/2021/formulas/chemistry/high-school/8avo0dsqp35upblr0mqjel4ws6rfh5knu7.png)
After the addition of HCl solution we have:
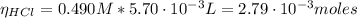
The HCl added will react with the conjugate base of the acetic acid:
H₃O⁺ + CH₃COO⁻ ⇄ CH₃COOH + H₂O
2.79x10⁻³moles 8.64x10⁻³moles
From the reaction of HCl with CH₃COO⁻ we have:
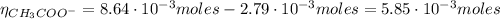
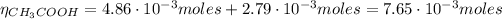
Finally, we can find the pH of the solution after the addition of HCl:
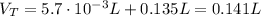
![pH = pKa + log(([CH_(3)COO^(-)])/([CH_(3)COOH]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/hd5fpicxme46zhywwc0kzmeqej1njjsban.png)
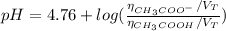
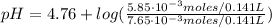

I hope it helps you!