Answer:
0.25M
Step-by-step explanation:
We can apply the following formula to find molarity ,since the Molarity (M) is the number of moles of solute per liter of solution.
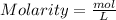
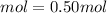 of KI(potassium iodide)
of KI(potassium iodide)
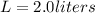
we can substitute the values to find the molarity
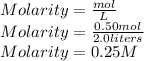
So the molarity of 2.0 liters of an aqueous solution that contains 0.50 mol of potassium iodide is 0.25M