Answer:
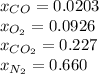
Step-by-step explanation:
Hello,
In this case, we consider the reaction:
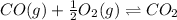
For which the law of mass action is expressed as:
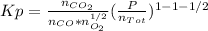
Whereas the exponents are referred to the stoichiometric coefficients in the chemical reaction. Moreover, in table A-28 (Cengel's thermodynamics) the natural logarithm of the undergoing reaction at 2600 K is 2.801, thus:
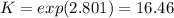
In such a way, in terms of the change
 the equilibrium goes:
the equilibrium goes:
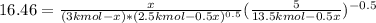
Hence, solving for
 :
:
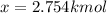
Thus, the moles at equilibrium:
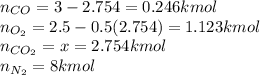
Finally the compositions:

Best regards.