The answer for the following question is explained below.
- Therefore the temperature that is require to fill the gas is 254.3 K
Step-by-step explanation:
Given:
no of moles (n) = 3.67 moles
volume of the gas (V) = 67.5 liters
pressure of the gas (P) = 115 k Pa
To solve:
Temperature of the gas (T)
We know;
P × V = n × R × T
Where;
P is pressure of the gas
V is the volume of the gas
n is the no of the moles
R is the universal gas constant
here the value of R(universal gas constant ) is 8.314 k Pa.
 /mole.K
/mole.K
We know;
115 × 67.5 = 3.67 × 8.3145 × T
T =
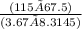
T =
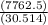
T = 254.39 K
Therefore the temperature that is require to fill the gas is 254.3 K