The answer for the question is answered below.
- 0.05 moles of air is present i the Erlenmeyer flask
Step-by-step explanation:
Given:
volume (v) =0.125 L
Pressure (P) =0.985 atm
temperature (t) =20°C =20 + 273 =293 K
To solve:
no of moles (n)
We know;
According to the Avagadros law;
P × V = n × R × T
0.985 × 0.125 = n × 0.0821 × 293
0.123125= n × 24.0553
n =
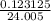
n = 0.05 moles
0.05 moles of air is present i the Erlenmeyer flask