Answer:
divide by molar mass (12.01 g)
Step-by-step explanation:
To find the number of moles of the carbon, divide by molar mass (12.01 g).
The number of moles of a substance is given as;
Number of moles =

To solve this problem;
since mass of carbon = 45g
Number of moles =
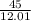 = 3.75moles
= 3.75moles