Answer:
The work per unit mass required for the liquid is 4.9 MJ
The work per unit mass required for the vapor is 0.639 MJ.
Step-by-step explanation:
Per unit mass of vapor, we have
p₁ = 1 bar = 0.1 MPa
p₂ = 50 bar = 5 MPa
mass of saturated water = 1 kg
Volume of saturated water = 1 m³
Since the water is incompressible, we have
Pump work = v×(p₂ - p₁) = 1 m³ × (5 MPa - 1 MPa) = 4.9 MJ
For the steam, we have. 1 kg steam at 1 bar we have the specific volume v₁ = 1.694 m³/kg also
1 kg steam at 50 bar has a specific volume v₂ = 0.039 m³/kg
Since the internal energy is constant, that is ΔU = 0, then, the temperature can be assumed to be constant. Therefore, we have
Work done in moving from p₁ to p₂ is given by;
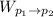 =
=
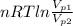 =
=
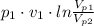 = 1.694 × 0.1 MPa ×
= 1.694 × 0.1 MPa ×
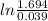 = 638855.89 J
= 638855.89 J
= 0.639 MJ.