Answer:
Equilibrium will go to backward direction
Step-by-step explanation:
The equilibrium reaction is :-
cis-2-butene ⇋ trans-2-butene
The equilibrium constant, Kp for the reaction = 3.40
The reaction quotient is:-
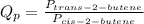
Given that :- Pressure of cis-2-butene = 10.00 atm
Pressure of trans-2-butene = 41.00 atm
Thus,
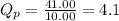
Qp > Kp, equilibrium will go to backward direction