Answer:
Final calculated molarity of the unknown acid be lower than the actual concentration.
Step-by-step explanation:
Volume required unknown acid = V= 25.0 mL
Volume actually measure was more than V that is = V'
V' > V
Molarity of the solution is the moles of compound in 1 Liter solutions.
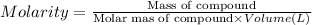
As we can see from the formula that molarity is inversely proportional to the volume of the solution.
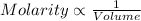
- Molarity of solution decreases with increase in volume
- Molarity of solution Increases with decrease in volume.
We have added more volume than required which will be increase the volume of solution and the molarity of the solution will lower than the actual value.