Answer: In the given chemical equation, the substance getting oxidized is magnesium metal.
Step-by-step explanation:
Oxidation reaction is defined as the chemical reaction in which an atom looses its electrons. The oxidation number of the atom gets increased during this reaction.
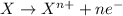
The chemical equation for the reaction of magnesium and hydrochloric acid follows:
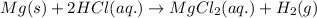
The half reaction for the above equation follows:
Oxidation half reaction:
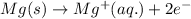
Reduction half reaction:
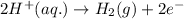
Hence, in the given chemical equation, the substance getting oxidized is magnesium metal.