819 ml is the volume the gas will occupy at 30.0°C.
Step-by-step explanation:
Data given:
initial volume of the gas V1 = 590 ml
initial temperature of the gas T1 = -55 degrees OR 218.15
final volume of the gas V2 = ?
final temperature of the gas T2 = 30 degrees OR 303.15
Charles' Law equation is used to calculate the volume of gas at 30 degrees from the data given in the question.
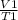 =
=
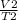
V2 =
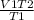
V2 =
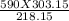
V2 = 819 ml
The final volume of the gas would be 819 ml.