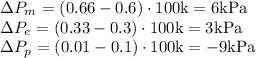Answer:
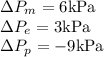
Step-by-step explanation:
mole fraction of propane after passing through the separator is
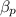
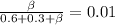
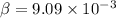
mole fractions of ethane
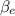 and methane
and methane
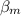 after passing through separator are:
after passing through separator are:

Change in partial pressures then can be written as:
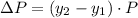 where
where
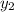 and
and
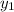 are mole fractions after and before passing through the separator
are mole fractions after and before passing through the separator
Hence,