Answer:
The final temperature of the system is 39⁰C
Step-by-step explanation:
Applying principle of conservation of heat energy;
Heat loss by a hot body = heat gained by a cold body
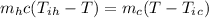
where;
Mh is the mass of the hot fluid = 330 g = 0.33 kg
Mc is the mass of the cold fluid = 855-g = 0.855 kg
Tih is the initial temperature of the hot fluid = 55°C
Tic is the initial temperature of the cold fluid = 10°C
T is the final temperature of the mixture
Substitute the given values and solve for T

Therefore, the final temperature of the system is 39⁰C