Answer:
10.88 g
Explanation:
We have:
[CH₃COOH] = 0.10 M
pH = 5.25
Ka = 1.80x10⁻⁵
V = 250.0 mL = 0.250 L
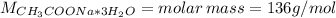
The pH of the buffer solution is:
![pH = pKa + log(([CH_(3)COONa*3H_(2)O])/([CH_(3)COOH]))](https://img.qammunity.org/2021/formulas/chemistry/college/wf3jur3wquofcn9zchea0w10e045j8ifsy.png) (1)
(1)
By solving equation (1) for [CH₃COONa*3H₂O] we have:
![log [CH_(3)COONa*3H_(2)O] = pH - pKa + log [CH_(3)COOH]](https://img.qammunity.org/2021/formulas/chemistry/college/lstehpwurg7q4dxsx3sezoo1kwbxolm8l4.png)
![log [CH_(3)COONa*3H_(2)O] = 5.25 - (-log(1.80 \cdot 10^(-5))) + log (0.10) = -0.495](https://img.qammunity.org/2021/formulas/chemistry/college/ysen0jfeuwwyrz1pdi62y6i4aml6pzwgw4.png)
![[CH_(3)COONa*3H_(2)O] = 10^(-0.495) = 0.32 M](https://img.qammunity.org/2021/formulas/chemistry/college/j6hqfifa1t0mmo34r4s2h9ytvc607ape7s.png)
Hence, the mass of the sodium acetate tri-hydrate is:
![m = moles*M = [CH_(3)COONa*3H_(2)O]*V*M = 0.32 mol/L*0.250 L*136 g/mol = 10.88 g](https://img.qammunity.org/2021/formulas/chemistry/college/kur27bpvqkjwkwjt5ndtsgy8v4qk5o2gvx.png)
Therefore, the number of grams of CH₃COONa*3H₂O needed to make an acetic acid/sodium acetate tri-hydrate buffer solution is 10.88 g.
I hope it helps you!