Answer:
a. 0.171M
b. 0.0938M
c. 0.284
d. 1.99atm
e. 1.88
Step-by-step explanation:
Hello,
In this case, for the given reaction whose balance should be corrected as:
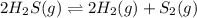
For which the law of mass action, in terms of the change
 due to stoichiometry and the reaction extent, turns out:
due to stoichiometry and the reaction extent, turns out:
![K=([H_2]_(eq)^2[S_2]_(eq))/([H_2S]_(eq)^2)](https://img.qammunity.org/2021/formulas/chemistry/college/3v160bqmheynnhssr0f1ams4k3m0luc9kq.png)
Thus, the initial concentration of hydrogen sulfide is:
![[H_2S]_0=(6.75g/(34g/mol))/(0.75L) =0.265M](https://img.qammunity.org/2021/formulas/chemistry/college/wsw6nseiaj6r6k718wynxf2nn4uy4sasg9.png)
Now, since the equilibrium amount of sulfur is given, the change
 due to equilibrium reaching is:
due to equilibrium reaching is:
![[S_2]_(eq)=x=(6.42x10^(-2)mol)/(0.75L)=0.0856M](https://img.qammunity.org/2021/formulas/chemistry/college/40pkdeeywhix21x0uyqufiimm60zgo3obd.png)
Therefore:
a. Equilibrium concentration of hydrogen:
![[H_2]_(eq)=2x=2*0.0856M=0.171M](https://img.qammunity.org/2021/formulas/chemistry/college/2aj76p8vnx6dwpln76ocvt5ffjkqqgairt.png)
b. Equilibrium concentration of hydrogen sulfide:
![[H_2S]_(eq)=0.265M-2x=0.265M-2*0.0856M=0.0938M](https://img.qammunity.org/2021/formulas/chemistry/college/oxsv08l9026e7izot9y2hrpznp73wij0hb.png)
c.) Equilibrium constant, Kc:
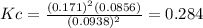
d.) Partial pressure of sulfur gas:
![p_(S_2)=[S_2]RT= 0.0856(mol)/(L)*0.082(atm*L)/(mol*K)*283K=1.99atm](https://img.qammunity.org/2021/formulas/chemistry/college/58knhzcnxbsf2gcuxmllxu3ertu0kzj6ib.png)
e. Kc, for the reaction:
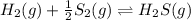
In that case, it equals the inverse halved initial reaction, whose modification is related as:
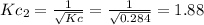
Best regards.