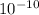Answer:
Filling in the blank:
Without doing any calculations (using tables) it is possible to determine that cobalt(II) carbonate (CoCO3) is more soluble than A, B, C, and cobalt(II) carbonate is less soluble than D. It is not possible to determine whether cobalt(II) carbonate is more or less soluble than by simply comparing Ksp values.
Step-by-step explanation:
Ksp (Solubility product constant) is a constant indicating the equilibrium between a solid and its respective ions in a solution. The higher the Ksp the more soluble or dissociation the compound is in water.
Using ksp table (at 25°C)
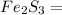 1.4 ×
1.4 ×
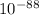
Zn3(PO4)2 = 9.1 ×
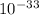
CrPO4 = 2.4 ×
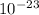
CaCO3 = 3.36 ×
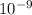
CoCO3 = 1.0 ×