Answer:
0.161 moles of Fe₂O₃
Step-by-step explanation:
Given data:
Mass of Fe = 18g
Unknown:
Number of moles = Fe₂O₃
Solution:
The number of moles of a substance is given as:
Number of moles =

To solve this problem, we should understand that oxygen gas is given in excess and iron is the limiting reactant.
The equation of the reaction is given below;
4Fe + 3O₂ → 2Fe₂O₃
First, find the number of moles of the known specie which is iron;
Number of moles of Fe =
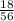 = 0.32mole
= 0.32mole
From the balanced reaction equation;
4 moles of Fe will produce 2 moles of Fe₂O₃
0.32 moles of Fe will produce
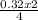 = 0.161 moles of Fe₂O₃
= 0.161 moles of Fe₂O₃