Answer:
3.72L
Step-by-step explanation:
Given parameters:
Initial volume V₁ = 3.9L
Condition = STP
Final temperature T₂ = -550°C
Final pressure P₂ = 880mmHg
Unknown:
Final volume V₂ = ?
Solution.
At standard temperature and pressure(STP), the:
Pressure = 1atm = 760mmHg
Temperature = 273K
Therefore, P₁ = 760mmHg
T₁ = 273K
The general gas law, is best to solve this problem. It is mathematically given as:
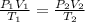
Let us take the units to the appropriate one;
-550°C = 273 + (-550) = -277K
Input the variables;
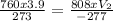
V₂ = 3.72L