Answer:
Reaction A has a higher net reaction rate
Step-by-step explanation:
Rate of electrochemical reaction rate per unit area =
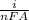
F = 96500 C
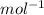 ( Faraday constant)
( Faraday constant)
n = number of moles of electrons per mole of reactant
A = Area of electrode
i = current generated
convert the current from Amperes to C
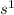
To calculate net reaction of reaction A
i = 5 c
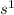
n = 2
A = 2
back to the equation
= 5 / (2 * 96500 * 2) = (1.3 * 10 ^ -5) mols^-1 cm^-2
To calculate net reaction of reaction B
i = 15 c
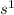
n = 3
A = 5
back to the equation
= 15 / ( 3 * 96500 * 5) = (1.036 * 10 ^ -5) mols^-1 cm^-2