Answer:
2.88x10⁻¹⁸ C
Step-by-step explanation:
The net charge is given by:
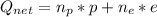
Where:
np: is the quantity of protons = 26
ne: is the quantity of electrons = 8
p: is the charge of protons = 1.6x10⁻¹⁹ C
e: is the charge of electrons = -1.6x10⁻¹⁹ C
Hence the net charge is:
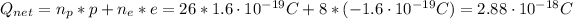
Therefore, the net charge is 2.88x10⁻¹⁸ C.
I hope it helps you!