Answer:
2.90 x 10⁻¹¹moldm⁻³
Step-by-step explanation:
Given parameters:
[H⁺] = 3.5 x 10⁻⁴mol/dm³
Unknown
[OH⁻] = ?
Solution;
The ionic product of water can be used to solve this problem. It has been experimentally determined to be 1 x 10⁻¹⁴mol² dm⁻⁶
[H⁺] [OH⁻] = 1 x 10⁻¹⁴
Therefore;
[OH⁻] =
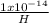 =
=
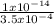 = 0.29 x 10⁻¹⁰moldm⁻³
= 0.29 x 10⁻¹⁰moldm⁻³
= 2.90 x 10⁻¹¹moldm⁻³