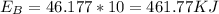Answer:
a
The rate of radiation of the energy is
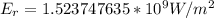
b
The irradiation is
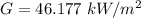
c
The amount of energy absorbed is
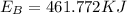
d
The oak Tree would catch fire because the temperature of the blast(7200 K) is higher than the flammability limit (650 K) of the oak tree and secondly the thickness is very small
Step-by-step explanation:
From the question we are told that
The temperature is
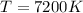
The diameter of the ball is
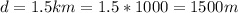
Hence the radius =
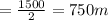
The total energy radiated can be mathematically represented as
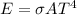
Where
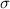 is the Stefan-Boltzmann constant
is the Stefan-Boltzmann constant
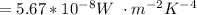
A is the area of a sphere =
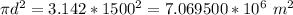
Substituting values we have
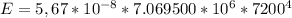

Now the state of the energy is mathematically represented as
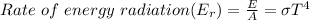
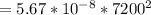
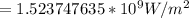
A sketch illustrating the b part of the question is shown on the first uploaded image
looking at the height at which the blast occurs(16km) as compared to the height of the wall we notice that the height of the wall is negligibly small
from the diagram x can be calculated as follows
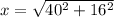
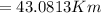
This value of x represents the radius of the blast(assuming it is spherical ) when it is at that wall
Now the irradiation G is mathematically represented as
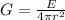
Here r = 43.0813 Km = 43.0813 × 1000 = 43081.3 m
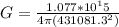
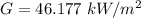
Generally the amount of energy absorbed can be mathematically represented as
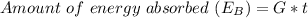
Where t is the time taken
Therefore