Answer:
2. Decreases
Step-by-step explanation:
From the combined concept of entropy and the second law of thermodynamics, it can be concluded that during the positive change in entropy, the degree of randomness of a system increases.
But in case of photosynthesis, we see a negative entropy change because in the process of photosynthesis plants take disordered things as
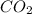 from the atmosphere and water from soil and finally make a highly ordered molecule Glucose, which is further used as a food product and source of energy by the plants. Thus it is clear that degree of randomness in decreasing in the process of photosynthesis and it is a negative change in entropy.
from the atmosphere and water from soil and finally make a highly ordered molecule Glucose, which is further used as a food product and source of energy by the plants. Thus it is clear that degree of randomness in decreasing in the process of photosynthesis and it is a negative change in entropy.