Answer:
1.19 g
Step-by-step explanation:
Given that:
Molecular weight of diene
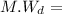 136 g/mol
136 g/mol
Molecular weight of Maleic Anhydride
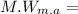 96 g/mol
96 g/mol
Mass of crude diene = 2.5 g
Percent of Composition for Peak A = 66%
Percent of Composition for Peak B = 22.66%
Percent of Composition for Peak C = 11.33%
Let us determine the amount of diene in the sample;
So, using
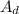 to represent the amount of diene; we have :
to represent the amount of diene; we have :
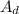 =
=

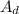 =
=
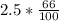
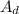 = 1.65 g
= 1.65 g
Using the limiting reagent equation to determine the amount of Maleic Anhydride
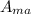
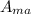 =
=
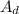
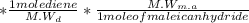
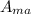 =
=
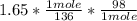
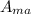 = 1.19 g
= 1.19 g
The grams of Maleic Anhydride used to react with 2.5 g sample of crude diene = 1.19 g