Answer:
Equilibrium concentration of Cl₂ = 0.013 molar
Equilibrium concentration of Cl = 0.0072 molar
Step-by-step explanation:
Cl ₂ ( g ) ⇄ 2 Cl ( g )
No. of moles of Cl₂(g) =
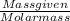
=
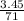
= 0.048 mole
Concentration of Cl₂ =
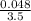 M = 0.013 molar
M = 0.013 molar
From the above equation we get
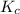 =
=
![([Cl]^(2) )/([Cl_(2) ])](https://img.qammunity.org/2021/formulas/chemistry/college/f2tiel9dceb4bwuojv0z231icp9kaimuuo.png)
[Cl]² = Kc X [Cl₂]
= 0.00376 x 0.013
= 5.22 x 10⁻⁵ molar
[Cl] = 0.0072 molar