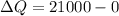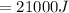Answer:
The thermal energy of the house is
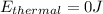
The magnitude of the energy transfer is
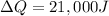
Step-by-step explanation:
From the question we are told that
The energy content is
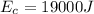
The energy through sunshine is
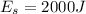
Generally the first law of thermodynamics can be mathematically represented as
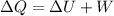
Where
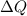 is the heat transferred
is the heat transferred
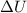 is the change in internal energy with is = 0J the temperature of the house did not change
is the change in internal energy with is = 0J the temperature of the house did not change
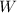 is the workdone which = 0J This because the volume of the air in the house did not change
is the workdone which = 0J This because the volume of the air in the house did not change
Therefore
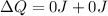
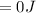
And this is equivalent to the thermal energy of the house
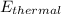
Therefore
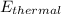 = 0J
= 0J
Now the heat transfer from outside to inside can be mathematically represented as
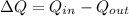
Now
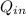 = 19000 + 2000
= 19000 + 2000
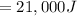
This because both the energy of the natural and the sunshine are supplied to the house
And
![Q_{out]](https://img.qammunity.org/2021/formulas/physics/college/2eaauxvus230d8bnnt6xtxvbt9n6aqieag.png) = 0 this is because there are no transfer of heat to the surrounding
= 0 this is because there are no transfer of heat to the surrounding
Hence