Answer:
(a)
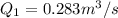
(b)
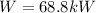
Step-by-step explanation:
Hello,
(a) Based on the inlet conditions, we see that such carbon dioxide has an ideal behavior, so the inlet specific volume is:
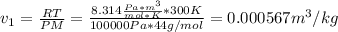
Thus, the volumetric flow rate turns out:
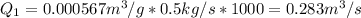
(b) Now, by writing and energy balance we have:
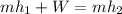
Thus, since the ideal gas enthalpy does not depend on the pressure, in Cengel's A-20 table, the corresponding enthalpies at 300K and 450 K are 9431kJ/kmol and 15483kJ/kmol whose values in kJ/kg are 214.34 kJ/kg and 351.89 kJ/kg respectively, thus:
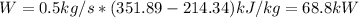
Best regards.