Answer:
See explanation.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
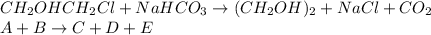
For which the differential equations in terms of the time variation are:
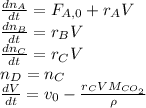
And the implicit equations:
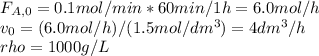
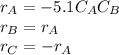
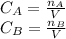
Thus, on the attached pictures you will find the conversion, concentrations, rate and volume profiles as well as the number of moles of ethylene glycol considering a maximum volume of 2500 dm³.
This is the code I used on matlab:
-
t=0;
na(1,1)=0;
nb(1,1)=1125;
nc(1,1)=0;
nd(1,1)=0;
V(1,1)=1500;
k=length(na);
C_A(1,1)=0;
C_B(1,1)=0.75;
x(1,1)=0;
t(1,1)=0;
r_A(k,1)=0;
while V(k,1)<2500
na(k+1,1)=na(k,1)+0.01*(6+r_A(k,1)*V(k,1));
nb(k+1,1)=nb(k,1)+0.01*(r_A(k,1)*V(k,1));
nc(k+1,1)=nc(k,1)+0.01*(-r_A(k,1)*V(k,1));
nd(k+1,1)=nd(k,1)+0.01*(-r_A(k,1)*V(k,1));
V(k+1,1)=V(k,1)+0.01*(4-(-r_A(k,1))*V(k,1)*44/1000);
r_A(k+1,1)=-5.1*C_A(k,1)*C_B(k,1);
C_A(k+1,1)=na(k+1,1)/V(k+1,1);
C_B(k+1,1)=nb(k+1,1)/V(k+1,1);
C_C(k+1,1)=nc(k+1,1)/V(k+1,1);
C_D(k+1,1)=nd(k+1,1)/V(k+1,1);
x(k+1,1)=(nb(1,1)-nb(k+1,1))/nb(1,1);
t(k+1,1)=t(k,1)+0.01;
k=k+1;
end
-
Best regards.