Answer : The half-life of the radioisotope is, 8.00 min
Explanation :
First we have to calculate the rate constant.
Expression for rate law for first order kinetics is given by:
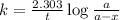
where,
k = rate constant = ?
t = time passed by the sample = 16 min
a = initial amount of the reactant = 4800
a - x = amount left after decay process = 1200
Now put all the given values in above equation, we get
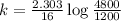
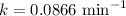
Now we have to calculate the half-life.
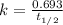
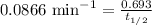
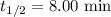
Therefore, the half-life of the radioisotope is, 8.00 min