We have 8.22
 1
1
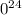 C atoms
C atoms
We have 4.11
 1
1
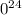 ethanol molecules
ethanol molecules
Step-by-step explanation:
Step 1: Data given
Molecular formula of ethanol =
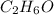
Mass of C = 164 grams
Molar mass C = 12.01 g/mol
Step 2: Calculate moles C
Moles C = mass C / molar mass C
Moles C = 164 grams / 12.01 g/mol
Moles C = 13.66 moles
Step 3: Calculate molecules of C
Atoms C = moles C
 number of Avogadro
number of Avogadro
Atoms C = 13.66 moles
 6.02
6.02
 1
1
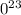 molecules/ mol
molecules/ mol
Atoms C = 8.22
 1
1
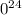 C atoms
C atoms
Step 4: Calculate molecules in the sample
For 1 mol
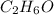 we have 2 moles C
we have 2 moles C
For 13.66 moles C we have 13.66/ 2 = 6.83 moles
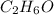
Molecules
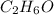 = 6.83 moles
= 6.83 moles
 6.02
6.02
 1
1
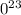 = 4.11
= 4.11
 1
1
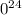 molecules.
molecules.