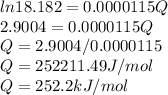Answer:
Activation energy for creep in this temperature range is Q = 252.2 kJ/mol
Step-by-step explanation:
To calculate the creep rate at a particular temperature
creep rate,
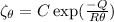
Creep rate at 800⁰C,
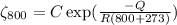
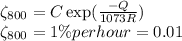
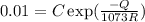 .........................(1)
.........................(1)
Creep rate at 700⁰C
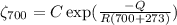
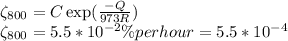
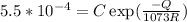 .................(2)
.................(2)
Divide equation (1) by equation (2)
![(0.01)/(5.5 * 10^(-4) ) = \exp[(-Q)/(1073R) -(-Q)/(973R) ]\\18.182= \exp[(-Q)/(1073R) +(Q)/(973R) ]\\R = 8.314\\18.182= \exp[(-Q)/(1073*8.314) +(Q)/(973*8.314) ]\\18.182= \exp[0.0000115 Q]\\](https://img.qammunity.org/2021/formulas/engineering/college/uvbnbfkxwpgdqawoh883u42ky6f1xpm44s.png)
Take the natural log of both sides