Answer: 41.5 mL
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
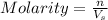
where,
n = moles of solute
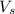 = volume of solution in L
= volume of solution in L
Given : 59.4 g of
 in 100 g of solution
in 100 g of solution
moles of
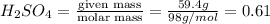
Volume of solution =
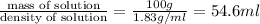
Now put all the given values in the formula of molality, we get
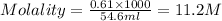
To calculate the volume of acid, we use the equation given by neutralisation reaction:

where,
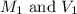 are the molarity and volume of stock acid which is
are the molarity and volume of stock acid which is

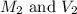 are the molarity and volume of dilute acid which is
are the molarity and volume of dilute acid which is

We are given:
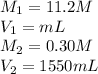
Putting values in above equation, we get:
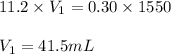
Thus 41.5 mL of the solution would be required to prepare 1550 mL of a .30M solution of the acid