Answer: The final volume of the gas is 1.45 L
Step-by-step explanation:
To calculate the amount of work done for an isothermal process is given by the equation:
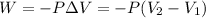
W = amount of work done = -145.0 J = 1.431 L.atm (Conversion factor: 1 L.atm = 101.33 J)
For expansion, work done is always negative
P = pressure = 783 Torr = 1.03 atm (Conversion factor: 1 atm = 760 torr)
 = initial volume = 60.7 mL = 0.0607 L (Conversion factor: 1 L = 1000 mL)
= initial volume = 60.7 mL = 0.0607 L (Conversion factor: 1 L = 1000 mL)
 = final volume = ?
= final volume = ?
Putting values in above equation, we get:
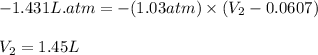
Hence, the final volume of the gas is 1.45 L