Answer:
-852 KJ
Explanation:
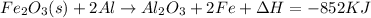
The above reaction is an exothermic reaction. Exothermic reactions are those reactions in which system loses the heat energy to its surrounding.
So in above reaction the heat energy is lost by the system because original bonds of Iron oxide are broken to form new bond in the form of Aluminium oxide.