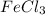Answer:
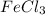
Step-by-step explanation:
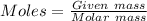
mass of Fe = 55.85 g
Molar mass of Fe = 55.85 g/mol
Moles of Fe = 55.85 / 55.85 = 1
mass of Cl = 106.5 g
Molar mass of Cl = 35.5 g/mol
Moles of Cl = 106.5 / 35.5 = 3
Taking the simplest ratio for Fe and Cl as:
1 : 3
The empirical formula is =