Answer:
4.48 grams of potassium hydroxide that the chemist must be weighing out.
Step-by-step explanation:
The pH of the KOH solution = 13
pH + pOH = 14
pOH = 14 - pH = 14 - 13 = 1
![pOH=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aptpm2b2equoweomw80psbpn50765hcb2n.png)
![1=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/e8w2mnmk4obqa4wejp0rszg28am14bfpew.png)
![[OH^-]=0.1 M](https://img.qammunity.org/2021/formulas/chemistry/college/w59ash4fml8y12yuvfy7q8u87bliytki1e.png)
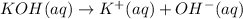
1 mole of hydroxide ions are obtained from 1 mole of KOH. Then 0.1 mole of hydroxide ions will be obtained from :
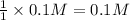 of KOH
of KOH
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](https://img.qammunity.org/2021/formulas/chemistry/high-school/xr4p44e7md4zj1cp15ien0t9yodzdaqgj6.png)
Volume of KOH solution = 800 mL = 0.800 L ( 1 mL = 0.001 L)
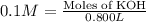
Moles of KOH = 0.1 M × 0.800 L = 0.08 mol
Mass of 0.08 moles of KOH :
0.08 mol × 56 g/mol = 4.48 g
4.48 grams of potassium hydroxide that the chemist must be weighing out.