Answer:
The value of entropy change for the process
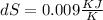
Step-by-step explanation:
Mass of the ideal gas = 0.0027 kilo mol
Initial volume
 = 4 L
= 4 L
Final volume
 = 6 L
= 6 L
Gas constant for this ideal gas ( R ) =
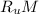
Where
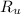 = Universal gas constant = 8.314
= Universal gas constant = 8.314
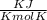
⇒ Gas constant R = 8.314 × 0.0027 = 0.0224
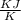
Entropy change at constant temperature is given by,
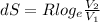
Put all the values in above formula we get,
![dS = 0.0224 log _(e) [(6)/(4)]](https://img.qammunity.org/2021/formulas/chemistry/college/8wcifc9c4a7as5r55hvfrxnmczhbn8890e.png)
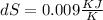
This is the value of entropy change for the process.