Answer:
1) v = 2.20 10⁵ m / s , 2) r = 4.86 10⁻³ m, r = 4.92 10⁻³ m
Step-by-step explanation:
1) A speed selector is a section where the magnetic and electrical forces have opposite directions, so
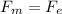
q v B = q E
v = E / B
V = E s
E = V / s
v = V / s B
v = 148 / (0.0016 0.42)
v = 2.20 10⁵ m / s
2) when the isotopes enter the spectrometer, we can use Newton's second law
F = m a
Acceleration is centripetal
a = v² / r
q v B = m v² / r
r = m v / qB
The mass of uranium 235 is
m = 3.903 10⁻²⁵ kg
The radius of this isotope is
r = 3,903 10⁻²⁵ 2.20 10⁵ / (92 1.6 10⁻¹⁹ 1.2)
r = 4.86 10⁻³ m
The mass of the uranium isotope 238 is
m = 238 a = 238 1.66 10-27 = 395.08 10-27 kg
The radius is
r = 395.08 10⁻²⁷ 2.20 10⁵ / (92 1.6 10⁻¹⁹ 1.2)
r = 4.92 10⁻³ m