Answer:
0.93 g of disodium ethylenediaminetetraacetate is required.
Step-by-step explanation:
Molarity of a solution = (Number of moles of solute in solution)/(Volume of solution in liter)
Here, disodium ethylenediaminetetraacetate is the solute.
Volume of solution = 0.250 L
Molarity of solution to be prepared = 0.010 M
So, number of moles of disodium ethylenediaminetetraacetate required =
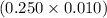 moles = 0.0025 moles
moles = 0.0025 moles
We know, number of moles = (mass)/(molar mass)
So, mass of disodium ethylenediaminetetraacetate required =
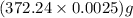 = 0.93 g
= 0.93 g
Hence, 0.93 g of disodium ethylenediaminetetraacetate is required.