Step-by-step explanation:
As we know that HCl is a stronger acid and NaOH is a stronger base.
And, HF and phenol are weaker acids having
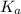 values of
values of
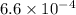 and
and
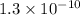 respectively.
respectively.
In the same way, methyl amine and pyridine are weaker bases with
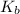 values of
values of
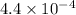 and
and
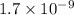 respectively.
respectively.
(i) Volume will be calculated as follows.
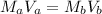
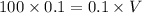
Therefore, volume of NaOH is 100 ml.
(ii) Similarly, volume of HCl is 100 ml.
(iii) For Methyl amine,
![[OH]^(-) = \sqrt{4.4 * 10^(-4) * 0.1}](https://img.qammunity.org/2021/formulas/chemistry/college/ochm25ll2re1ztp8uf97ke19c5nvbxpdzg.png)
=
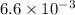
And as,
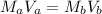
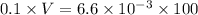
Hence, the volume of HCl is 6.6 ml.
(iv) For HF,
![[H]^(+) = \sqrt{6.6 * 10^(-4) * 0.1}](https://img.qammunity.org/2021/formulas/chemistry/college/gq7yuo2b3poq9qrwq1f2bfvx1xit5qlb3k.png)
=
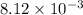
As,
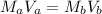
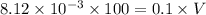
Hence, the volume of NaOH added is 8.12 ml.
Therefore, we can conclude that the increasing order of volums of given titrant is (i) = (ii) > (iv) > (iii).