The question is incomplete, here is the complete question:
If 65 mL of sulfuric acid and 25 mL of sodium hydroxide were mixed and the solution had a density of 1.01 g/mL, What is the heat of the calorimeter in kJ given the temperature change of the above equation is -5.5 K. You may assume the solution has a heat capacity of 4.180 J/gK. Express your final answer in kJ and with 2 decimal places
Answer: The heat of the calorimeter is 2.09 kJ
Step-by-step explanation:
To calculate the mass of solution, we use the equation:
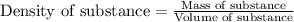
Density of solution = 1.01 g/mL
Volume of solution = [65 + 25] mL = 90 mL
Putting values in above equation, we get:
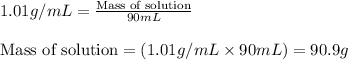
To calculate the heat released by the reaction, we use the equation:
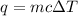
where,
q = heat released
m = mass of solution = 90.9 g
c = heat capacity of solution = 4.180 J/g.K
 = change in temperature = -5.5 K
= change in temperature = -5.5 K
Putting values in above equation, we get:
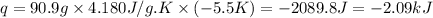
Heat released by the solution will be equal to the heat absorbed by the calorimeter.
Sign convention of heat:
When heat is absorbed, the sign of heat is taken to be positive and when heat is released, the sign of heat is taken to be negative.
Heat absorbed by the calorimeter = -(-2.09) = 2.09 kJ
Hence, the heat of the calorimeter is 2.09 kJ