Answer:
Both can be correct is steam exits as superheated steam.
Step-by-step explanation:
The model for the turbine is created by using the First Law of Thermodynamics:
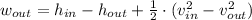
Specific enthalpies for both states are presented below:
State 1 (Superheated Steam)
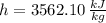
State 2 (Saturated Vapor)
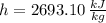
The specific work of turbine is:
![w_(out) = 3562.10\,(kJ)/(kg) - 2693.10\,(kJ)/(kg)+(1)/(2)\cdot [(60\,(m)/(s) )^(2)-(89.4\,(m)/(s) )^(2)] \cdot ((1\,kJ)/(1000\,J) )](https://img.qammunity.org/2021/formulas/engineering/college/1gxtqfinpqkzaz3ff5ut1hig8x17buznfk.png)
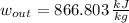
Both can be correct is steam exits as superheated steam.