Answer:
The answers to the questions are;
a. The temperature T where the work outputs of the two engines are equal is 700 K.
b. The temperature T where the efficiencies of the two engines are equal is 600 K.
Step-by-step explanation:
To solve the question, we note that
T₁ = 927 °C = 1200.15 K
T₃ = 27 °C = 300.15 K
We are required to calculate T the output temperature of the first engine
(i) When the work outputs of the two engines are equal we have
We put the the work output to be = W
Whereby the work done by the first engine is given by the work done in Carnot cycle which is -∑ W = ∑ Q
Then A receives heat at Q₁ at 1200.15 K and rejects heat at Q₂ at T K therefore W
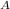 = Q₁ - Q₂
= Q₁ - Q₂
Similarly B receives heat at Q₂ at T K and rejects heat at Q₃ at 300.15 K
therefore W
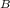 = Q₂ - Q₃
= Q₂ - Q₃
Where W
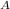 = W
= W
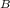
Q₁ - Q₂ = Q₂ - Q₃ Dividing both sides by Q₁, we have
(Q₁ - Q₂)/Q₁ = (Q₂ - Q₃)/Q₁
1 - Q₂/Q₁ = Q₂/Q₁ - Q₃/Q₁
1 - T/T₁ = T/T₁ -T₃/T₁
Which gives 1+T₃/T₁ = 2 ×T/T₁
T = T₁ /2×(1+T₃/T₁ ) = 1200.15/2×(1+300.15/1200.15) = 750.15 K
T = 750.15 K ≈ 750 K
b. When the efficiencies of the two engines are equal we have
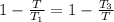 which gives
which gives
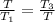 therefore T² = T₃×T₁ = (1200.15 K) × (300.15 K) = 360225.0225 K²
therefore T² = T₃×T₁ = (1200.15 K) × (300.15 K) = 360225.0225 K²
T =
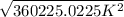 = 600.187 K ≈ 600 K
= 600.187 K ≈ 600 K