Required data to solve this problem:
- Avogadro's number: 6.022x10²³
- Avogadro's number = 1 mole
- Molar mass of calcium: 40.078 g/mol
This is a unit analysis problem wherein we are given Avogadro's number and we're trying to output a mass in grams. Molar mass can be used as a conversion factor, since molar mass is in units of grams per mole. Since we know that Avogadro's number is equal to one mole, we can set up a unit analysis to go from Avogadro's number to grams, with moles as the middleman, so to speak.
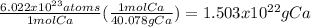
The moles cancel and the answer is Avogadro's number divided by 40.078.
Answer:
1.503x10²² grams