Answer: Strong bases are good electrolytes because they completely ionize in water.
Step-by-step explanation:
Weak bases are those substances which dissociate partially to give ions when dissolved in water.
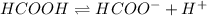
Strong bases are those substances which dissociate completely to give ions when dissolved in water.
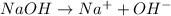
Strong bases are good electrolytes as they completely dissociate to give ions which help in the conduction of electrical current.