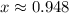Answer:
Step-by-step explanation:
The correct formula for the potential energy between two atoms in a particular molecule is:
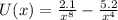
Where
 is the distance.
is the distance.
According to the definitions of potential energy and work, as well as the Work-Energy Theorem and the Principle of Energy Conservation. The relation between that and related force is:
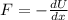
The function is derived in terms of distance:
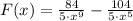
Then, it is needed to find at least of x so that F(x) equals to 0.
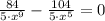
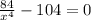
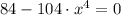
![x=\sqrt[4]{(84)/(104) }](https://img.qammunity.org/2021/formulas/physics/college/cjwvs8d9spzi16csye1l6xa2w2skcni2mk.png)