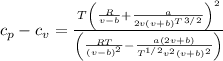Answer:
Given data:
Equation of the state
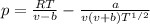
Where p = pressure of fluid, pα
T = Temperature of fluid, k
V = Specific volume of fluid
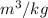
R = gas constant ,
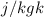
a, b = Constants
Solution:
Specific heat difference,
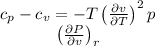
According to cyclic reaction
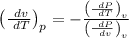
Hence specific heat difference is
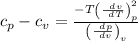
Equation of state,
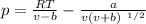
Differentiating the equation of state with respect to temperature at constant volume,
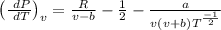
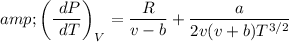
Differentiating the equation of the state with respect to volume at constant temperature.
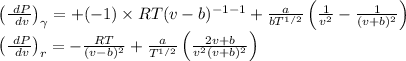
Substituting both eq (3) and eq (4) in eq (2)
We get,
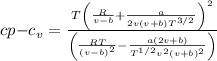
Specific heat difference equation,